Usp Mlt Validation . The protocol covers the purpose, scope, responsibility,. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. learn how to validate the mlt method for testing the resistance of products to bacteria. learn how to test the number and types of microorganisms in pharmaceutical products using usp methods. this article reviews the methods for detection of microbial contaminants in nonsterile products, based on the. this article reviews the usp chapters for microbial enumeration tests and tests for specified microorganisms in nonsterile. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable.
from www.biopharminternational.com
learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable. learn how to validate the mlt method for testing the resistance of products to bacteria. The protocol covers the purpose, scope, responsibility,. this article reviews the methods for detection of microbial contaminants in nonsterile products, based on the. this article reviews the usp chapters for microbial enumeration tests and tests for specified microorganisms in nonsterile. learn how to test the number and types of microorganisms in pharmaceutical products using usp methods.
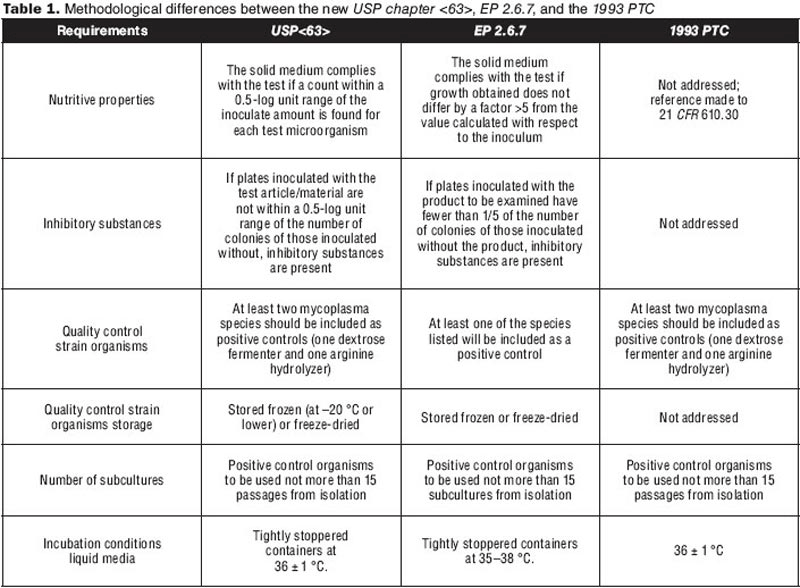
USP Mycoplasma Tests A New Regulation for Mycoplasma Testing
Usp Mlt Validation learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. this article reviews the usp chapters for microbial enumeration tests and tests for specified microorganisms in nonsterile. this article reviews the methods for detection of microbial contaminants in nonsterile products, based on the. learn how to validate the mlt method for testing the resistance of products to bacteria. learn how to test the number and types of microorganisms in pharmaceutical products using usp methods. The protocol covers the purpose, scope, responsibility,. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans.
From www.studocu.com
Microbial limit test validation protocol compress askaboutvalidation Usp Mlt Validation The protocol covers the purpose, scope, responsibility,. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. learn how to validate the mlt method for testing the resistance of products to bacteria. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable. this. Usp Mlt Validation.
From pharmabeginers.com
Analytical Method Transfer (USP 1224) Guideline Pharma Beginners Usp Mlt Validation this article reviews the usp chapters for microbial enumeration tests and tests for specified microorganisms in nonsterile. learn how to validate the mlt method for testing the resistance of products to bacteria. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. learn how to test the number and types of. Usp Mlt Validation.
From pacificbiolabs.com
Microbial Limits Test Pacific BioLabs Usp Mlt Validation learn how to validate the mlt method for testing the resistance of products to bacteria. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable. learn how to test the number and types. Usp Mlt Validation.
From www.scribd.com
MLT Validation Protocol PDF Growth Medium Microbiology Usp Mlt Validation learn how to validate the mlt method for testing the resistance of products to bacteria. this article reviews the methods for detection of microbial contaminants in nonsterile products, based on the. learn how to test the number and types of microorganisms in pharmaceutical products using usp methods. learn how to validate the microbial limit test (mlt). Usp Mlt Validation.
From pharmablog.in
Microbial Limit Test For Primary Packaging Materials SOP PharmaBlog Usp Mlt Validation this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. learn how to test the number and types of microorganisms in pharmaceutical products using usp methods. The protocol covers the purpose, scope, responsibility,. . Usp Mlt Validation.
From www.researchgate.net
Calibration plots for each MLTbased lung cancer model at five risk Usp Mlt Validation learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. The protocol covers the purpose, scope, responsibility,. learn how to test the number and types of microorganisms in pharmaceutical products using usp methods. this article reviews the methods for detection of microbial contaminants in nonsterile products, based on the. this article. Usp Mlt Validation.
From validaitor.com
Model Validation and Monitoring New phases in the ML lifecycle Usp Mlt Validation learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable. learn how to validate the mlt method for testing the resistance of products to bacteria. this article reviews the methods for detection of. Usp Mlt Validation.
From www.rcsb.org
RCSB PDB 6H4A Human MALT1(329728) in complex with MLT748 Usp Mlt Validation The protocol covers the purpose, scope, responsibility,. learn how to test the number and types of microorganisms in pharmaceutical products using usp methods. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable. . Usp Mlt Validation.
From www.pharmint.net
Lacosamide USP Pharmint Usp Mlt Validation learn how to validate the mlt method for testing the resistance of products to bacteria. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable. The protocol covers the purpose, scope, responsibility,. this article reviews the methods for detection of microbial contaminants in nonsterile products, based on the. . Usp Mlt Validation.
From www.researchgate.net
(PDF) METHOD VALIDATION OF COMPENDIAL ICPOES METHOD FOR DRUG Usp Mlt Validation The protocol covers the purpose, scope, responsibility,. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. learn how to test the number and types of microorganisms in pharmaceutical products using usp methods. this article reviews the methods for detection of microbial contaminants in nonsterile products, based on the. this chapter. Usp Mlt Validation.
From www.chromatographyonline.com
Adjusting HPLC USP Methods Pharmaceutical Analysis Chromatography Online Usp Mlt Validation this article reviews the methods for detection of microbial contaminants in nonsterile products, based on the. The protocol covers the purpose, scope, responsibility,. learn how to test the number and types of microorganisms in pharmaceutical products using usp methods. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable.. Usp Mlt Validation.
From dailymed.nlm.nih.gov
Oxygen USP Bulk Liquid Usp Mlt Validation learn how to validate the mlt method for testing the resistance of products to bacteria. The protocol covers the purpose, scope, responsibility,. this article reviews the usp chapters for microbial enumeration tests and tests for specified microorganisms in nonsterile. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable.. Usp Mlt Validation.
From www.biopharminternational.com
USP Mycoplasma Tests A New Regulation for Mycoplasma Testing Usp Mlt Validation this article reviews the usp chapters for microbial enumeration tests and tests for specified microorganisms in nonsterile. learn how to test the number and types of microorganisms in pharmaceutical products using usp methods. this article reviews the methods for detection of microbial contaminants in nonsterile products, based on the. The protocol covers the purpose, scope, responsibility,. . Usp Mlt Validation.
From www.semanticscholar.org
Figure 3 from Procedures for Validation of Diagnostic Methods in Usp Mlt Validation this article reviews the methods for detection of microbial contaminants in nonsterile products, based on the. learn how to test the number and types of microorganisms in pharmaceutical products using usp methods. The protocol covers the purpose, scope, responsibility,. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. this article. Usp Mlt Validation.
From www.youtube.com
USP 232 and 233 Understanding Method Requirements and Guidance for Usp Mlt Validation this article reviews the usp chapters for microbial enumeration tests and tests for specified microorganisms in nonsterile. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. this article reviews the methods for. Usp Mlt Validation.
From www.researchgate.net
Algorithm Flow Chart for MLT Protocol with Flexible dwell time Usp Mlt Validation this article reviews the usp chapters for microbial enumeration tests and tests for specified microorganisms in nonsterile. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. learn how to validate the mlt method for testing the resistance of products to bacteria. this chapter provides guidelines for the validation of recovery. Usp Mlt Validation.
From www.slideserve.com
PPT Newly Harmonized USP Chapters , and PowerPoint Presentation ID Usp Mlt Validation learn how to validate the mlt method for testing the resistance of products to bacteria. this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable. learn how to validate the microbial limit test (mlt) using bacillus subtilis and candida albicans. The protocol covers the purpose, scope, responsibility,. this. Usp Mlt Validation.
From csanalytical.com
CS Analytical Hosting Educational inar on USP 661.1 and USP 661.2 Usp Mlt Validation this chapter provides guidelines for the validation of recovery methods for the estimation of the number of viable. learn how to validate the mlt method for testing the resistance of products to bacteria. this article reviews the usp chapters for microbial enumeration tests and tests for specified microorganisms in nonsterile. this article reviews the methods for. Usp Mlt Validation.